Chemical Principles AAA 751
What is an Element ?
An element is a substance that cant be broken into simpler component using chemical means.
Modern Definition :
Each element or atom consists of a nucleus made up of protons and neutrons and a number of electrons in shells or orbits around the nucleus. "A substance consisting of atoms of the same Atomic Number"
There are the same number of electrons as protons in an atom.
Exercise:
Ans :
Symbols & Formulae
Each element is assigned a symbol and where an element can exist as a single atom the 1 is assumed and were it exists as a molecule (more than one atom joined) the number is appended
|
Atomic Number |
Symbol |
Name |
Atomic Weight |
Formulae |
|
1 |
H |
Hydrogen |
1.00794 |
H2 |
|
2 |
He |
Helium |
4.002602 |
He |
|
3 |
Li |
Lithium |
6.941 |
Li |
|
4 |
Be |
Beryllium |
9.012182 |
Be |
|
5 |
B |
Boron |
10.811 |
B |
|
6 |
C |
Carbon |
12.0107 |
C |
|
7 |
N |
Nitrogen |
14.0067 |
N2 |
|
8 |
O |
Oxygen |
15.9994 |
O2 |
|
9 |
F |
Fluorine |
18.9984032 |
F2 |
|
10 |
Ne |
Neon |
20.1797 |
Ne |
|
11 |
Na |
Sodium |
22.989770 |
Na |
|
12 |
Mg |
Magnesium |
24.3050 |
Mg |
|
13 |
Al |
Aluminium |
26.981538 |
Al |
|
14 |
Si |
Silicon |
28.0855 |
Si |
|
15 |
P |
Phosphorus |
30.973761 |
P4 |
|
16 |
S |
Sulfur |
32.065 |
S8 |
|
17 |
Cl |
Chlorine |
35.453 |
Cl2 |
|
18 |
Ar |
Argon |
39.948 |
Ar |
|
19 |
K |
Potassium |
39.0983 |
K |
|
20 |
Ca |
Calcium |
40.078 |
Ca |
Mixture:
A mixture contains two or more substances separable by physical means - there is no chemical bond.
Examples:
| Mixture | Contains | Method of separation |
| Sulfur / Iron filings | magnetic separation. | |
| Salt Solution | Sodium Chloride / Water | distillation, osmosis |
| Carbon / Copper II Sulfate. | Carbon is not soluble in water but CuSO4 is so the mixture is heated in water and the carbon filtered off. The filtrate is evaporated to leave the copper sulfate crystals. | |
| Brass | Copper and Zinc | |
| Milk | water fat sugar protein vitamins | |
| Granite | Quartz biotite feldspar |
Questions:
Which of the following are mixtures?
Ans:
Compounds
Certain chemical materials have a tendency to combine to form a different substance called a compound. This tendency is called chemical bonding and is related to the number of electrons in the outer orbit or shell. In many cases heat or special circumstances are required for the materials to combine. This is also true for substances to break apart.
Some Common Compounds
| Name | Formula |
| Water | 22 4 3 11 12 HO |
| Sulfur Dioxide | SO2 |
| Ammonia | NH3 |
| Silicon Dioxide - Sand | SiO2 |
| Sodium Chloride - table salt | NaCl |
| Calcium Carbonate - Limestone | CaCO 3 |
| Copper II Sulfate (anhydrous) | CuSO4 |
| Copper II Sulfate Crystal | CuSO4.5H2O |
| Methane | CH4 |
| Hydrochloric Acid | HCl |
| Nitric Acid | HNO3 |
| Sulfuric Acid | H2SO4 |
| Sugar | C12H22O11 |
| Magnesium Oxide | MgO |
Electron Dot Diagrams
The Hydrogen Bond
?? Find Background on Linus Pauling.
Linus Pauling found that when a Hydrogen atom Between two strongly electronegative atom something interesting happens:
The Hydrogen is attracted (in fact bonded) to the two strongly electronegative atoms.
Find Diagrams.
What are these strongly electronegative atoms? Fluorine > Oxygen > Nitrogen > Sulfur in order of the bond strength.
Effects of Hydrogen bonding on compounds:
Weak Intramolecular Forces
Intermolecular forces are forces of interaction between molecules. e.g. The forces between molecular crystals are weak forces. these weak forces are responsible for the low melting and boiling points of molecular substances.
| Type of Interaction | Approx. Energy KJ/mol |
| Intermolecular | |
| Van der Waals (dipole/dipole, dispersion) | 0.1 - 10 |
| Hydrogen Bonding | 10 - 40 |
| Chemical Bonding | |
| Ionic | 100 - 1000 |
| Covalent | 100 - 1000 |
Dipole / Dipole forces exist amoung polar molecules e.g. HCl resulting from the tendency of polar molecules to arrange themselves such that the positive end of one molecule is near the negative end of another. This can be regular as in a solid or random and fluid as in a liquid.
Find Diagram
Since the charges on the dipole is small the attraction between the molecules is also . This explains why HCl m.p. is -114 C and NaCl.
Dispersion forces : Non polar molecules like He H2 or F2 have no dipole force, yet such substances do liquefy. Therefore another type of intermolecular force exists. This weak force is called "dispersion" or "London" force (after Fritz London). There are instantaneous temporary dipoles resulting from there being more electrons on one or the other side of an atom. These dipoles that arise as the positions of the electrons about the nucleus vary give rise to an attraction. Dispersion forces tend to increase with molecular mass since there are more electrons present.
| Noble Gases | Halogens | |||||
| Formula | Mol Mass | m.p. C° | Formula | Mol Mass | m.p. C° | |
| He | 4 | -270 | F2 | 38 | -220 | |
| Ne | 20 | -249 | Cl2 | 71 | -101 | |
| Ar | 48 | -189 | Br2 | 160 | -7 | |
| Kr | 84 | -159 | I2 | 254 | 114 |
Dispersion forces account for the rising boiling point s with increasing carbon chain length. With increasing length greater polarization occurs.
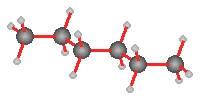 |
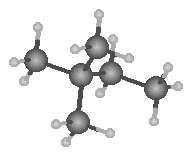 |
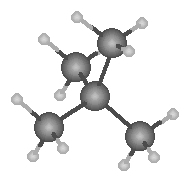 |
| Pentane | 2- methylbutane | 2,2-dimethylpropane |
| b.p. 36.1°C | b.p. 27.9°C | b.p. 9.5°C |
Links
The Poon-Mundy Computer Demonstrations
http://www.knowledgebydesign.com/tlmc/tlmc.html